Adapted from Kang et al. Experimental & Molecular Medicine 2018.
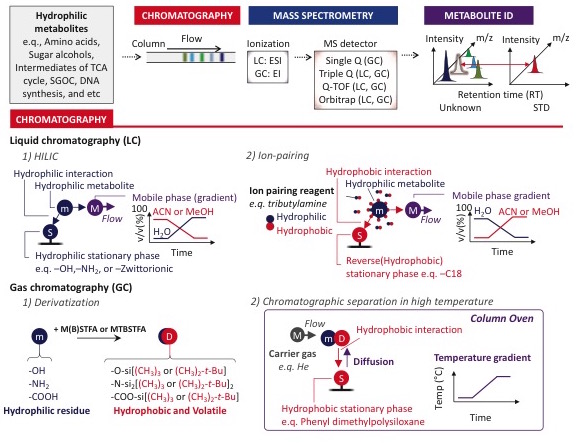
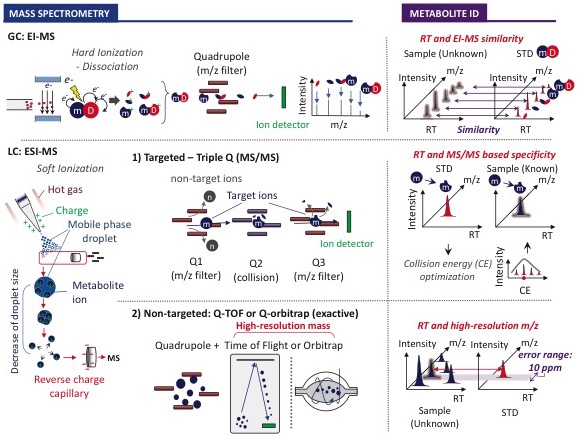
Analysis of sulfur containing metabolites by mass spec
Our laboratory utilizes a combination of gas chromatography mass spectrometry (GC-MS), targeted liquid chromatography mass spectrometry (LC-MS/MS, with triple quadrupole) and non-targeted LC-MS (with orbitrap) to profile and quantify metabolite changes in cells, fluids and tissues. With these technologies we perform both metabolite quantitation and flux analysis using stable isotope labeled substrates. However, thiol-containing metabolites including cysteine and glutathione are very labile and require special derivatization during sample preparation to prevent their oxidation. We have optimized the derivatization and quantification of sulfur containing metabolites in various tissues, cell types and fluids for the study of sulfur metabolism (Kang et al. eLife 2019).
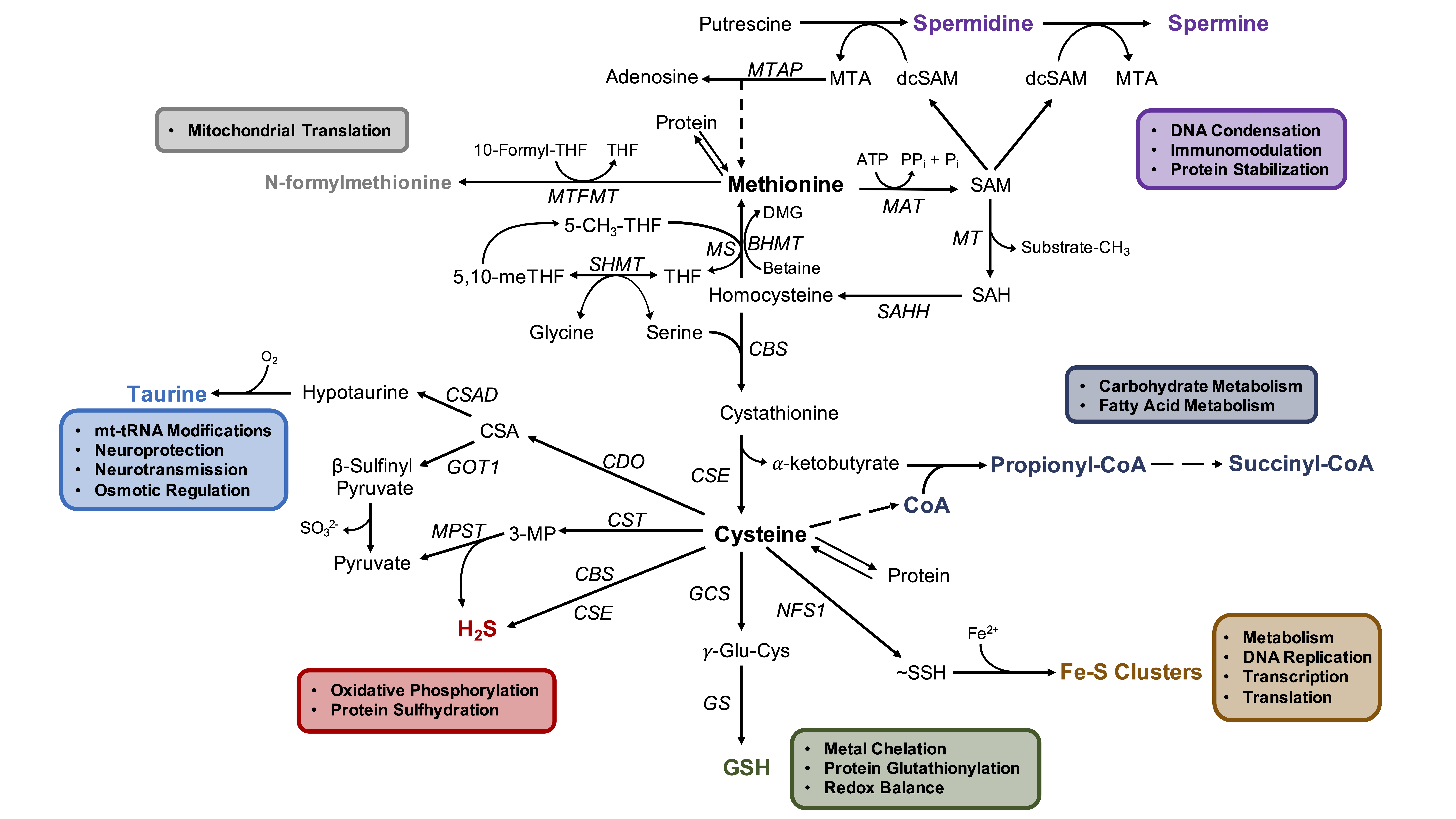
Adapted from Ward and DeNicola. Int Rev Cell Mol Biol. 2019;347:39-103. doi: 10.1016/bs.ircmb.2019.05.001.
Tracing the source and fate of sulfur in vivo
By delivering stable isotope labeled serine, methionine and cystine via the jugular catheter to mice, we are investigating how various tissues and tumors that arise within these tissues obtain cysteine and use it to synthesize downstream metabolites. We find that de novo cysteine synthesis capacity is present in healthy liver but absent in most healthy tissues. Consequently, tumors from genetically engineered mouse models that arise in these tissues also lack de novo cysteine synthesis capacity (lung adenocarcinoma, small cell lung cancer, pancreatic ductal carcinoma) or retain the de novo synthesis capacity of the parental tissue (hepatocellular carcinoma). In contrast, all healthy tissues and tumors derived from these tissues are capable of taking up cyst(e)ine to incorporate it into downstream metabolites like glutathione (Yoon et al. Cancer Research 2023). We are currently evaluating the fate of cysteine in these tumor models and other sources of cysteine within the tumor microenvironment.
The essentiality of sulfur-dependent processes in tumor initiation and progression
Cysteine plays an essential role in many NRF2-regulated processes. However, little is known about how different cellular compartments respond to cystine starvation and how cysteine is prioritized for cysteine-dependent processes. One such process is iron-sulfur (Fe-S) cluster synthesis, which requires cysteine-derived sulfur. Fe-S clusters are highly sensitive to ROS, which are detoxified by NADPH-dependent enzymes in the mitochondria. We found that the mitochondrial NADPH-generating enzyme nicotinamide nucleotide transhydrogenase (NNT) significantly enhances tumor formation and aggressiveness in mouse models of lung tumor initiation and progression by preventing mitochondrial Fe-S cluster oxidation to maintain mitochondrial function (Ward et al., J Exp Med 2020). Interestingly, loss of NNT does not influence global mitochondrial antioxidant capacity, suggesting that NNT serves a specific role in mitigating the oxidation of critical Fe-S cofactors in lung cancer and represents an attractive target for therapy.
The compartmentalization of cysteine metabolism in cancer
We have found that cysteine starvation results in the immediate cessation of cytosolic glutathione synthesis (Kang et al. Cell Metab 2021), but how the mitochondria deal with cysteine starvation is less clear. Interestingly, cysteine-dependent synthesis of Fe-S clusters is maintained in the mitochondria of lung cancer cells long after glutathione synthesis is impaired, resulting in the maintenance of mitochondrial function and redox state. Moreover, we find that mitochondrial glutathione sustains Fe-S synthesis via CHAC1-mediated catabolism of glutathione within the mitochondria. Disruption of CHAC1 results in a failure to maintain Fe-S protein activity. Surprisingly, disrupting Fe-S cluster synthesis or mitochondrial electron transport protects against the induction of ferroptosis under cysteine starvation, suggesting that the preservation of mitochondrial function is antagonistic to survival under starved conditions (Ward et al. Nat Commun 2024). Overall, our results demonstrate that mitochondria preserve their function at the expense of overall cellular health, which promotes ferroptosis when cysteine is limiting.
Open questions: