Cancers
Garg SK, Welsh EA, Fang B, Hernandez YI, Rose T, Gray J, Koomen JM, Berglund A, Mulé JJ, Markowitz J. Multi-Omics and Informatics Analysis of FFPE Tissues Derived from Melanoma Patients with Long/Short Responses to Anti-PD1 Therapy Reveals Pathways of Response. Cancers. 2020; 12(12):3515. https://doi.org/10.3390/cancers12123515
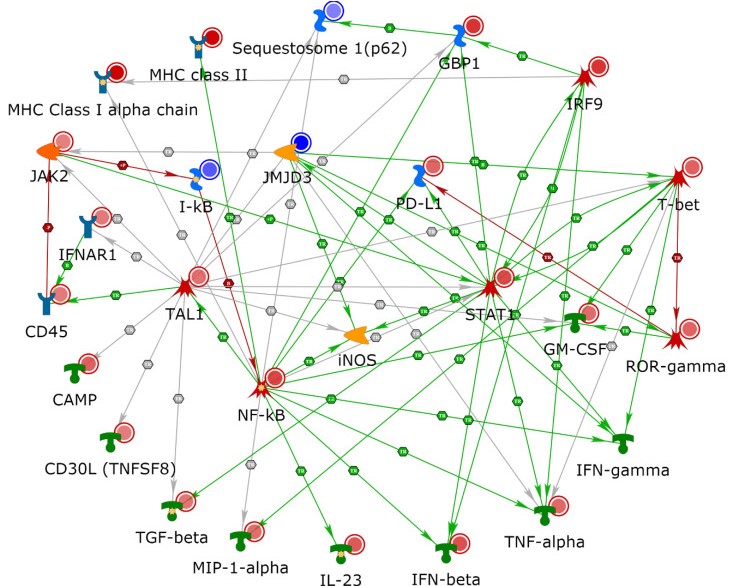
Anti-PD-1 based immune therapies are thought to be dependent on antigen processing and presentation mechanisms. To characterize the immune-dependent mechanisms that predispose stage III/IV melanoma patients to respond to anti-PD-1 therapies, we performed a multi-omics study consisting of expression proteomics and targeted immune-oncology-based mRNA sequencing. Formalin-fixed paraffin-embedded tissue samples were obtained from stage III/IV patients with melanoma prior to anti-PD-1 therapy. The patients were first stratified into poor and good responders based on whether their tumors had or had not progressed while on anti-PD-1 therapy for 1 year. We identified 263 protein/gene candidates that displayed differential expression, of which 223 were identified via proteomics and 40 via targeted-mRNA analyses. The downstream analyses of expression profiles using MetaCore software demonstrated an enrichment of immune system pathways involved in antigen processing/presentation and cytokine production/signaling. Pathway analyses showed interferon (IFN)-γ-mediated signaling via NF-κB and JAK/STAT pathways to affect immune processes in a cell-specific manner and to interact with the inducible nitric oxide synthase. We review these findings within the context of available literature on the efficacy of anti-PD-1 therapy. The comparison of good and poor responders, using efficacy of PD-1-based therapy at 1 year, elucidated the role of antigen presentation in mediating response or resistance to anti-PD-1 blockade.
Cancer Immunology, Immunotherapy
Bunch BL, Kodumudi KN, Scott E, Morse J, Weber AM, Berglund AE, Pilon-Thomas S, Markowitz J. Anti-tumor efficacy of plasmid encoding emm55 in a murine melanoma model. Cancer Immunol Immunother. 2020;69(12):2465-2476. doi:10.1007/s00262-020-02634-4
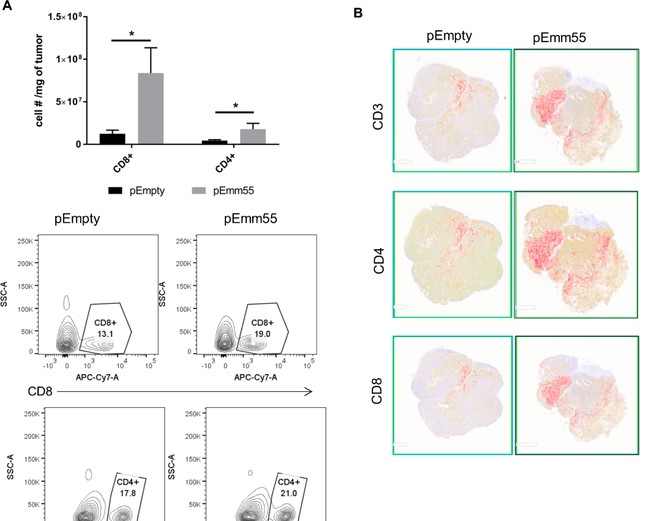
Emm55 is a bacterial gene derived from Streptococcus pyogenes (S. pyogenes) that was cloned into a plasmid DNA vaccine (pAc/emm55). In this study, we investigated the anti-tumor efficacy of pAc/emm55 in a B16 murine melanoma model. Intralesional (IL) injections of pAc/emm55 significantly delayed tumor growth compared to the pAc/Empty group. There was a significant increase in the CD8+ T cells infiltrating into the tumors after pAc/emm55 treatment compared to the control group. In addition, we observed that IL injection of pAc/emm55 increased antigen-specific T cell infiltration into tumors. Depletion of CD4+ or CD8+ T cells abrogated the anti-tumor effect of pAc/emm55. Combination treatment of IL injection of pAc/emm55 with anti-PD-1 antibody significantly delayed tumor growth compared to either monotherapy. pAc/emm55 treatment combined with PD-1 blockade enhanced anti-tumor immune response and improved systemic anti-tumor immunity. Together, these strategies may lead to improvements in the treatment of patients with melanoma.
Frontiers in Immunology
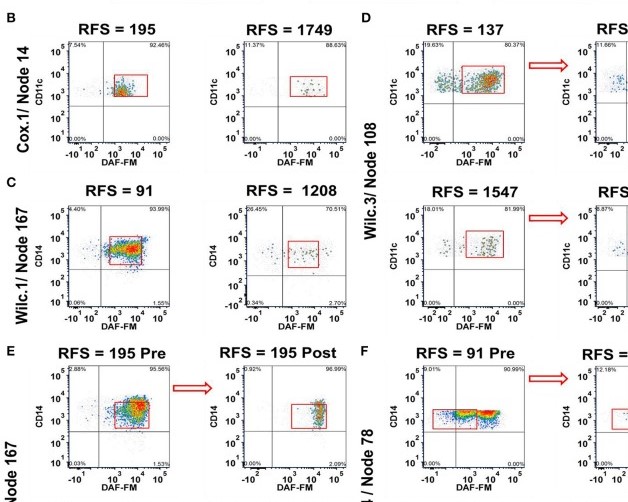
Phenotyping of immune cell subsets in clinical trials is limited to well-defined phenotypes, due to technological limitations of reporting flow cytometry multi-dimensional phenotyping data. We developed a multi-dimensional phenotyping analysis tool and applied it to detect nitric oxide (NO) levels in peripheral blood immune cells before and after adjuvant ipilimumab co-administration with a peptide vaccine in melanoma patients. We analyzed inhibitory and stimulatory markers for immune cell phenotypes that were felt to be important in the NO analysis. The pipeline allows visualization of immune cell phenotypes without knowledge of clustering techniques and to categorize cells by association with relapse-free survival (RFS). Using this analysis, we uncovered the potential for a dichotomous role of NO as a pro- and anti-melanoma factor. NO was found in subsets of immune-suppressor cells associated with shorter-term (≤ 1 year) RFS, whereas NO was also present in immune-stimulatory effector cells obtained from patients with significant longer-term (> 1 year) RFS. These studies provide insights into the cell-specific immunomodulatory role of NO. The methods presented herein can be applied to monitor the pro- and anti-tumor effects of a variety of immune-based therapeutics in cancer patients.