Our laboratory focuses on the rational design and synthesis of drug-like small molecules that can modulate specific cellular signaling pathways with an emphasis on protein–protein interactions and target specificity. Medicinal Chemistry, organic synthesis, and biological chemistry are of primary importance for our studies. We initiate our individual studies based on the molecular recognition between two proteins, develop new chemistry tools and techniques for the rational design, synthesis and characterization of selective protein–protein interaction inhibitors, and apply these newly established tools and techniques to tackle important and challenging therapeutic targets. Technique core in our laboratory is fragment-based de novo design.
There are four training components in the Ji research lab: (1) Computer Modeling and Bioinformatic Analysis; (2) Organic Synthesis and Compound Characterizations; (3) Biochemical Studies; and (4) Cell-Based Studies.
A. Development of New Techniques and Tools to Design, Synthesize, and Characterize Protein–Protein Interaction Inhibitors
- Design of small-molecule scaffolds to mimic the binding mode of α-helical hot spots.
α-Helices paly a major role in mediating protein–protein interactions. We observed that the side chains of helical hot spots at the same position tended to be placed in the same spatial areas when forming protein–protein complexes (See Hoggard et al. J. Am. Chem. Soc. 2015, 137 (38), 12249–12260). Small-molecule scaffolds themselves can mimic the positioning of hydrophobic side chains of α-helical hot spots are being designed and synthesized in the Ji laboratory. These scaffolds will be applied to design potent and selective small-molecule inhibitors for α-helix mediate protein–protein interactions.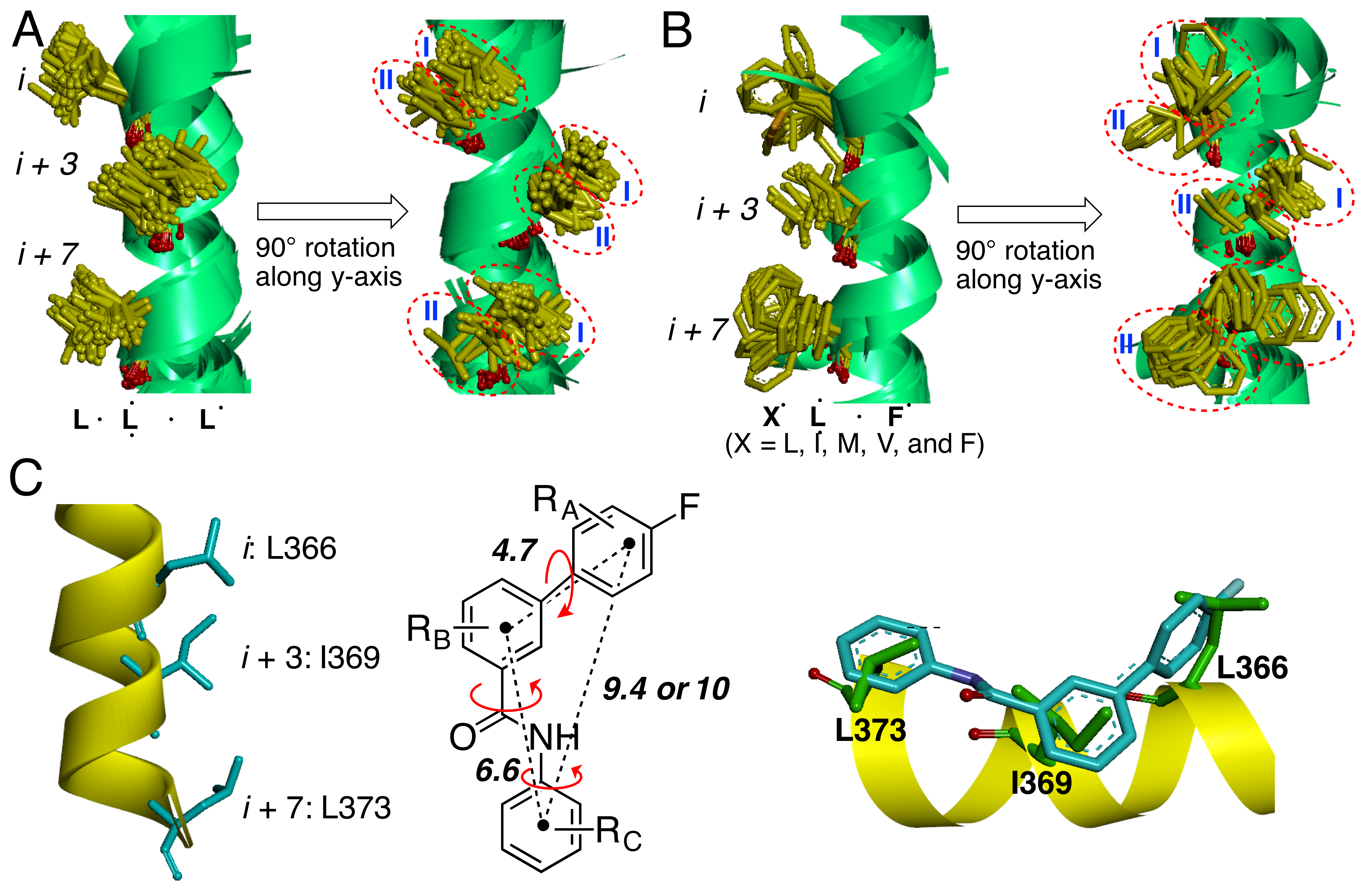
- Development of new synthetic methods.
A benzaldehyde mediated photoredox reaction for cross-dehydrogenative coupling was developed to Cα-arylate amides and ethers using a variety of five- and six-membered electron-deficient heteroarenes (See Zhang, Teuscher & Ji. Chem. Sci. 2016, 7 (3), 2111–2118.). The reaction proceeded smoothly with two household 23W CFL bulbs as energy source under metal-free conditions.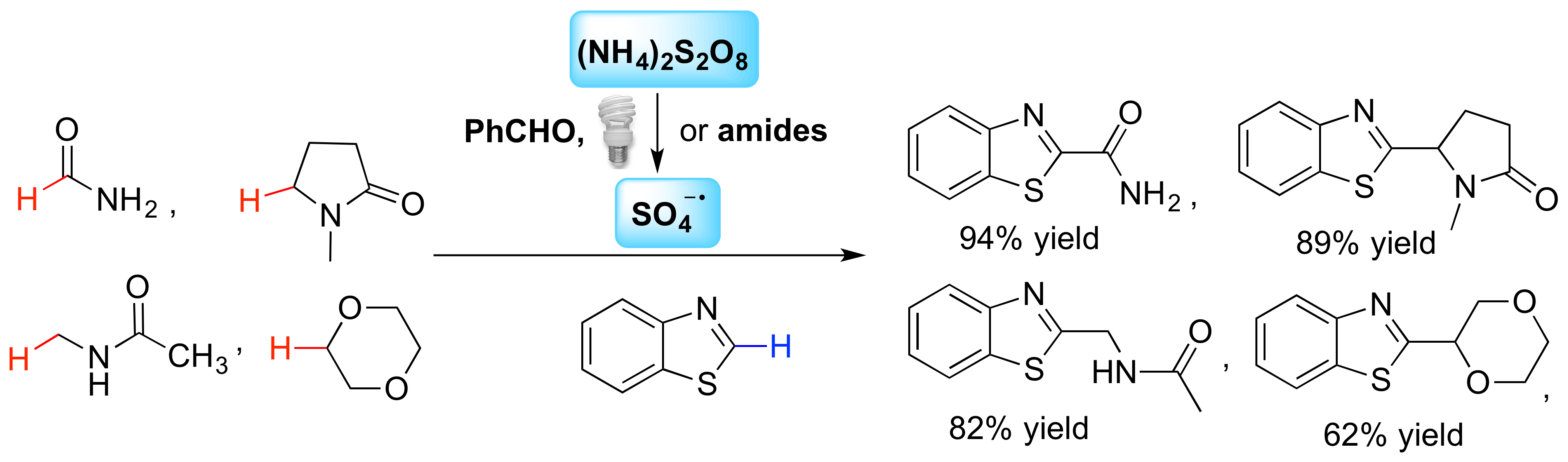
B. Design and Synthesis of Selective Inhibitors for Protein–Protein Interactions
Developmental pathways, including Notch, Hedgehog and Wnt, have emerged as important targets for the development of new anti-cancer or anti-fibrotic drugs. Inhibitors of Notch and Hedgehog are already in clinical trials. However, Wnt has far lagged behind. The lack of progress in identifying small-molecule inhibitors specific for the Wnt signaling pathway is largely due to the fact that protein–protein interactions dominate in this pathway, which has been challenging for drug discovery. Based on new fragment-based de novo design techniques established in our laboratory, small-molecule inhibitors with novel scaffolds are designed and synthesized.
- Design and synthesis of small molecules to selectively inhibit β-catenin/T-cell factor protein–protein interactions.
The β-catenin/T-cell factor (Tcf) protein–protein interaction is a downstream effector of the canonical Wnt signaling pathway. The aberrant formation of this complex drives the initiation and progression of many cancers and fibroses. The beta-catenin/cadherin and beta-catenin/adenomatous polysis coli (APC) interactions, on the other hand, are essential for cell–cell adhesion and maintain a low level of free β-catenin in normal cells. Beta-catenin uses the same interface to interact with Tcf, cadherin and APC. We design and synthesize selective small-molecule inhibitors for β-catenin/Tcf interactions and study the biology that is related with selective small-molecule inhibitors.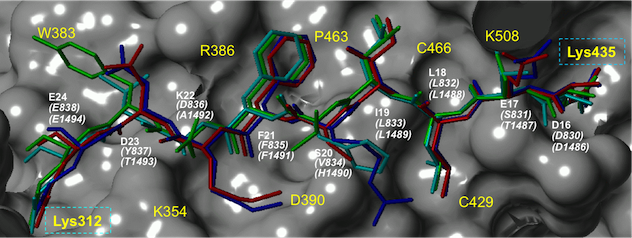
- Design and synthesis of small molecules to selectively inhibit β-catenin/B-cell lymphoma 9 protein–protein interactions.
The β-catenin/B-cell lymphoma 9 (BCL9) protein–protein interaction is unique for the canonical Wnt signaling pathway. The crystallographic analysis reveals that β-catenin interacts with BCL9 through an α-helical/α-helical interface which is also used by the β-catenin/cadherin interaction. We design and synthesize selective small-molecule inhibitors specific for the β-catenin/BCL9 interaction and study the biology that is related with selective small-molecule inhibitors.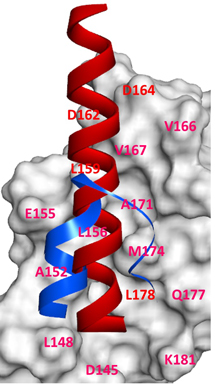
- Development of natural product building blocks for peptidomimetics.
Natural selection and evolution has led to an inherited evolutionary advantage to natural compounds. Natural product building blocks can be excellent starting points for fragment library design to mimic the biological functions of peptides. We identify interesting natural product building blocks and incorporate them into the sequence of native protein partners to mimic their biological functions. The challenge for this project is the synthesis of highly functionalized compounds.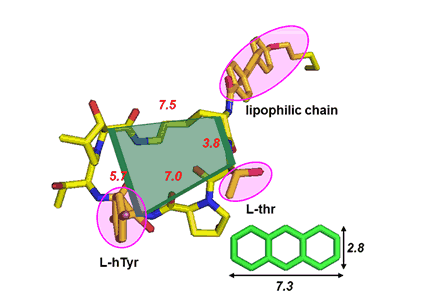
A graduate student or a postdoctoral fellow in this group is expected to receive training in structure-based inhibitor design, medicinal chemistry, synthetic organic chemistry, computer modeling, chemical biology, and molecular and cell biology.
