(Presented as a poster at the 2022 Annual Meeting of the American Society of Preventive Oncology)
Authors:
John Charles A. Lacson1*, Youngchul Kim2, Richard G. Roetzheim3, Steven K. Sutton2, Susan T. Vadaparampil3, Peter A. Kanetsky1
Affiliations:
1 Department of Cancer Epidemiology, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, US
2 Department of Biostatistics and Bioinformatics, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, US
3 Department of Family Medicine, Morsani College of Medicine, University of South Florida, Tampa, FL, US
4 Department of Health and Behavioral Outcomes, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, US
* Poster presenter, email: JohnCharles.Lacson@moffitt.org
Introduction
- Advances in genomic technologies and the emergence of precision medicine and prevention has provided the general populace affordable direct-to-consumer genetic testing (DTC-GT) that can interrogate genetic susceptibility to a variety of conditions and diseases, including cancers1.
- Companies offering DTC-GT operate on the assumption that test results can encourage modifications in behavior and lifestyle that can decrease disease risk2.
- Intervention trials in precision prevention have also provided genetic test results directly to participants together with genetics-driven prevention guidelines3, 4 and have been moderately successful in changing preventive behavior, especially in the realm of skin cancer prevention5-7.
- Retention of genetic risk information may motivate adoption and maintenance of preventive behaviors.
- No recall or inaccurate recall can jeopardize sharing of genetic risk information one’s health providers to implement screening and follow-up procedures8, and with other members of the family, who may opt to get tested themselves or seek related healthcare4.
- In this study, participants of a previous precision prevention trial that provided genetic risk information on MC1R, an established melanoma susceptibility gene9 were asked to recall their genetic risk after 6 and 12 months.
Methods
Participants and Setting
- Briefly, study participants were recruited between September 2015 to September 2018 from two university-associated primary care clinics in west-central Florida. Participants were non-Hispanic, White, at least 18 years of age, fluent in English, and did not report of a full-body skin examination within the past year or a personal history of melanoma.
- Participants were required to have limited melanoma risk phenotypes based on questions about hair color, tendency to burn, tendency to tan, and freckling.
- Participants completed a baseline questionnaire, provided a saliva sample for isolation of DNA and sequencing of MC1R, and were randomized in 1:1 ratio within MC1R risk stratum (average or higher) to receive precision prevention or generic prevention materials (standard).
- Participants randomized to the intervention arm were mailed precision prevention materials that included the participant’s MC1R risk category (average or higher) and information about interpretation of their risk category using both text and visual formats, contained within a packet that also included facts and figures about melanoma and genetics-based prevention guidelines.
- Participants randomized to the standard arm were mailed publicly available generic prevention materials (without genetic information).
- Within two weeks of mailing prevention materials, trained research staff followed up with participants by telephone and/or email to confirm the receipt of mailed materials and answer questions prompted by the participants. Research staff did not proactively remind or reiterate precision information provided in the precision prevention materials. After communicating with the participant (75.8%) or after three unsuccessful attempts, a summary letter was mailed that restated their MC1R risk category and recapitulated prevention guidelines.
Questionnaire assessments
- The baseline instrument, which participants completed before receiving precision (or standard) prevention materials, elicited information on age, sex, education, marital status, health literacy10, health numeracy11, family history of melanoma, skin cancer, and other cancers, and various psychosocial measures, including recent worry and concern about melanoma, absolute and comparative chance of getting melanoma, perceived severity, response efficacy, self-efficacy, cancer worry12, 13, and Impact of Event scale14.
- The baseline questionnaire also assessed eleven prevention activities over the past 12 months, taken from a standardized survey of sun exposure and sun protection behaviors 15.:
- number of hours spent outside between 10 a.m. and 4 p.m. separately for weekdays and weekends
- number of red or painful sunburns
- frequency (never, rarely, sometimes, often, always) of each of five sun protection behaviors:
- wearing a shirt with sleeves
- wearing a hat
- wearing sunglasses
- seeking shade or using an umbrella while outside
- using sunscreen
- frequency (never, rarely, sometimes, often, always) of intentional outdoor tanning; 9) number of intentional indoor tanning occurrences;
- skin examination (yes/no) performed by a health provider; and 11) number of skin examinations performed by oneself or partner.
- Six and 12 months after baseline, participants were asked again to report on the psychosocial measures and eleven prevention activities.
- An assessment of the prevention materials also was obtained at six months by asking participants to report the amount of intervention materials read (none, a few, some, most, all), believability and clarity of materials, and intention to change preventive behavior. The latter three variables were measured on a scale of 1 to 7, with 7 representing the highest value.
Risk recall
- At six and 12 months after baseline, participants in the intervention arm were asked to recall their MC1R genetic risk category (“Yes, it was average risk”; “Yes, it was high risk”; or “No, I don’t recall”).
- Risk recall over the 12-month follow up period was categorized as
- correct: participants who correctly recalled risk at each returned follow-up
- misremembered: participants who gave the incorrect risk category at either the 6- or 12-month assessment
- did not recall: participants who responded “No, I don’t recall” at each returned follow-up
- correct once: participants who correctly recalled risk at one follow-up but responded with “No, I don’t recall” at the other.
- Because of the small number (n=57 (5.0%)) of participants categorized as “correct once,” this category was combined with “correct” for all analyses.
Benchmarks of study engagement
- Indicator variables were created to capture participant completion of the telephone follow-up, and one or both of the 6- and 12-month follow-up questionnaires. These variables, along with the amount of intervention materials read, were used as proxy measures of study engagement.
Statistical analyses
- Predictors of risk recall
- Potential predictors of genetic risk recall included baseline variables, psychosocial measures, measures of prevention materials assessed at the 6-month follow-up, and benchmarks of study engagement. The univariate association of each variable with risk recall was assessed using appropriate statistical tests (chi-square, t-, Wilcoxon, Kruskal-Wallis, and ANOVA tests).
- Variables with p<0.20 were included in a backwards stepwise selection using Akaike Information Criterion (AIC)16.
- For average-risk participants, the one individual who misremembered their genotype was grouped with those who did not recall, and the outcome was modeled as binary (correct/correct once versus did not recall/misremembered).
- For higher-risk participants, the outcome was modeled as multinomial (correct/correct once versus did not recall versus misremembered). We also repeated analyses including psychosocial constructs measured at baseline instead of at six months.
- Analyses were conducted using R software (ver 4.1.0, R Foundation for Statistical Computing, Vienna, Austria, RRID:SCR_001905) and RStudio (ver 1.4.1717, RStudio Team, Boston, MA, RRID:SCR_000432).
- Assessment of intervention effects stratified by risk recall
- Within stratum defined by risk recall and MC1R risk category, intervention effects were estimated using previously published methods6. Briefly, the intervention effect on each of 10 prevention activities was determined using a generalized estimating equation (GEE) after adjusting for imbalanced baseline variables, predictors of missingness for return of the 6- and 12-month assessments, predictors of outcome, and the baseline measure of the outcome.
- Weekday and weekend sun exposure, number of sunburns, and frequency of outdoor intentional tanning were assumed to have a normal distribution and were modelled using the canonical identity link function. Similar models were used for a summary outcome variable for sun protection behaviors that was equal to the number of behaviors practiced often or always.
- The five sun protection behaviors were also examined individually as binary outcomes (often or always vs. sometimes, rarely, or never) and modelled using a logit link function. Intervention effects for skin exams were estimated using a logistic regression model that included only those who did not have a skin exam at baseline and returned the 12-month follow-up; the model included outcome predictors and imbalanced baseline variables.
- Dichotomous variables were derived to indicate ever having a skin examination over the study period. Skin exams conducted by a health professional, by self/partner, or either, were analyzed separately using multivariate logistic regression to estimate odds ratios (ORs). Participants who reported having a skin exam at baseline (n=25 by a health professional, n=117 by self/partner, n=135 by either or both) were excluded from these analyses. Participants who did not return their 12-month survey were excluded from these analyses, precluding the inclusion of missing predictors in the model.
- Analyses were conducted using SAS ver. 9.4 (SAS Institute, Cary, NC, USA, RRID:SCR_008567).
Results
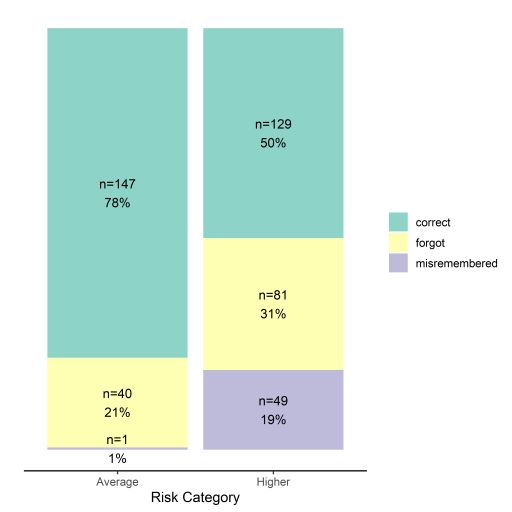
Among participants at higher risk (n=259), 50% correctly recalled, 31% did not recall, and 19% misremembered. Higher risk participants were over three times more likely (OR=3.61, 95% CI: 2.38-5.56) to misremember or not recall than average risk participants
|
Baseline and 6-month predictors of not recalling or misremembering (vs. correctly recalling) genetic risk by risk category.a
|
|
Outcome
|
Predictor
|
Odds ratio
|
95% confidence interval
|
|
MC1R average risk
|
|
Did not recall or misrememberedb
|
Marital status
|
|
|
|
|
|
Single
|
1.00
|
REF
|
|
|
|
Married, civil union or domestic partnership
|
10.20
|
2.24
|
63.61
|
|
|
Separated, divorced, or widowed
|
2.55
|
0.09
|
36.31
|
|
|
Family history of non-melanoma skin cancer
|
0.16
|
0.03
|
0.67
|
|
|
Clarity
|
0.55
|
0.32
|
0.90
|
|
|
Amount of prevention materials read
|
0.42
|
0.20
|
0.82
|
|
|
Telephone follow-up incomplete
|
15.79
|
2.82
|
109.80
|
|
|
Chance of getting melanoma (absolute)
|
|
|
|
|
|
Unlikely
|
1.00
|
REF
|
|
|
|
No idea
|
26.0
|
6.38
|
135.72
|
|
|
Likely
|
13.70
|
2.13
|
102.56
|
|
MC1R higher risk
|
|
Did not recall
|
Education level
|
0.63
|
0.45
|
0.89
|
|
|
Amount of prevention materials read
|
0.56
|
0.36
|
0.85
|
|
|
Chance of getting melanoma (comparative)
|
0.43
|
0.27
|
0.68
|
|
Misremembered
|
Education level
|
1.44
|
0.92
|
2.26
|
|
|
Amount of prevention materials read
|
0.72
|
0.45
|
1.15
|
|
|
Chance of getting melanoma (comparative)
|
0.25
|
0.15
|
0.40
|
a Predictors determined from logistic regression models using backwards stepwise selection.
b Only one participant at MC1R average risk misremembered their genetic risk.a Predictors determined from logistic regression models using backwards stepwise selection.
| Primary prevention outcome measures at baseline and post-intervention and intervention effects by genetic risk recall and MC1R risk category. |
| Outcomes |
Correct Recall
Beta/OR
|
Did not recall or
Misremembered
Beta/OR
|
| MC1R average risk |
| Continuous outcomesa |
|
|
| Weekday hours |
0.11 |
0.14 |
| Weekend hours |
0.11 |
0.10 |
| Sun protection behaviors |
0.15 |
0.06 |
| Sunburns |
-0.05 |
-0.09 |
| Outdoor intentional tanning |
0.03 |
0.22 |
| Cancer worry |
-0.03 |
0.08 |
| Binary outcomesb |
|
|
| Wearing a hat often or always |
1.34 |
1.93 |
| Seeking shade or using umbrella often or always |
1.19 |
1.26 |
| Wearing a shirt with sleeves often or always |
1.09 |
0.97 |
| Wearing sunglasses often or always |
1.10 |
0.48 |
| Wearing sunscreen often or always |
1.05 |
0.37* |
| Skin Examsc |
|
|
| Professional |
0.87 |
0.93 |
| Self/partner |
1.25 |
0.84 |
| Professional or self/partner |
1.08 |
0.91 |
| MC1R higher risk |
| Continuous outcomesa |
|
|
| Weekday hours |
0.02 |
0.004 |
| Weekend hours |
0.03 |
-0.01 |
| Sun protection behaviors |
0.28* |
0.07 |
| Sunburns |
0.02 |
0.05 |
| Outdoor intentional tanning |
0.002 |
-0.002 |
| Cancer worry |
-0.03 |
-0.10* |
| Binary outcomesb |
|
|
| Wearing a hat often or always |
1.33 |
0.51* |
| Seeking shade/using umbrella often or always |
1.56* |
1.27 |
| Wearing a sleeved shirt often or always |
1.65* |
1.38 |
| Wearing sunglasses often or always |
1.04 |
1.19 |
| Wearing sunscreen often or always |
0.88 |
0.62 |
| Skin Examsc |
|
|
| Professional |
1.01 |
0.98 |
| Self/partner |
0.85 |
0.73 |
| Professional or self/partner |
1.23 |
0.94 |
a Intervention effects are reported as beta-coefficients.
b Intervention effects are reported as odds ratios (ORs).
c Analyses of skin exams included only those that returned the 12-month questionnaire and did not have skin exams at baseline. Odds ratios of the association between arm and odds of having a skin exam during the follow-up period were estimated from a multivariate logistic regression.
* These intervention effects are statistically significant (p<0.05)
Conclusions
-
Recipients of genetic tests results conveying higher risk may have defensive reactions that would hinder their understanding and retention of genetic risk information.
-
Participants who correctly recalled their genetic risk had generally stronger intervention effects, indicating better adoption of prevention guidelines.
-
Future studies should use this information to develop strategies that can increase study engagement and circumvent defensive reactions to improve genetic risk recall and consequently improve prevention outcomes.
References
- Roberts MC, Fohner AE, Landry L, et al. Advancing precision public health using human genomics: examples from the field and future research opportunities. Genome Med. Jun 1 2021;13(1):97. doi:10.1186/s13073-021-00911-0
- Kilbride MK, Bradbury AR. Evaluating Web-Based Direct-to-Consumer Genetic Tests for Cancer Susceptibility. JCO Precis Oncol. 2020;4doi:10.1200/PO.19.00317
- Frieser MJ, Wilson S, Vrieze S. Behavioral impact of return of genetic test results for complex disease: Systematic review and meta-analysis. Health Psychol. Dec 2018;37(12):1134-1144. doi:10.1037/hea0000683
- Stewart KFJ, Wesselius A, Schreurs MAC, Schols A, Zeegers MP. Behavioural changes, sharing behaviour and psychological responses after receiving direct-to-consumer genetic test results: a systematic review and meta-analysis. J Community Genet. Jan 2018;9(1):1-18. doi:10.1007/s12687-017-0310-z
- Smit AK, Allen M, Beswick B, et al. Impact of personal genomic risk information on melanoma prevention behaviors and psychological outcomes: a randomized controlled trial. Genet Med. Aug 12 2021;doi:10.1038/s41436-021-01292-w
- Lacson JCA, Doyle SH, Qian L, et al. A Randomized Trial of Precision Prevention Materials to Improve Primary and Secondary Melanoma Prevention Activities among Individuals with Limited Melanoma Risk Phenotypes. Cancers (Basel). Jun 23 2021;13(13):3143. doi:10.3390/cancers13133143
- Lacson JCA, Doyle SH, Del Rio J, et al. A Randomized Clinical Trial of Precision Prevention Materials Incorporating MC1R Genetic Risk to Improve Skin Cancer Prevention Activities Among Hispanics. Cancer Research Communications. 2022;2(1):28-38. doi:10.1158/2767-9764.Crc-21-0114
- Cohidon C, Cardinaux R, Cornuz J, et al. May direct-to-consumer genetic testing have an impact on general practitioners' daily practice? a cross-sectional study of patients' intentions towards this approach. BMC Fam Pract. Apr 26 2021;22(1):79. doi:10.1186/s12875-021-01428-6
- Pasquali E, Garcia-Borron JC, Fargnoli MC, et al. MC1R variants increased the risk of sporadic cutaneous melanoma in darker-pigmented Caucasians: a pooled-analysis from the M-SKIP project. Int J Cancer. Feb 1 2015;136(3):618-31. doi:10.1002/ijc.29018
- Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. Sep 2004;36(8):588-94.
- Figueroa JD, Garcia-Closas M, Humphreys M, et al. Associations of common variants at 1p11.2 and 14q24.1 (RAD51L1) with breast cancer risk and heterogeneity by tumor subtype: findings from the Breast Cancer Association Consortium. Hum Mol Genet. Dec 1 2011;20(23):4693-706. doi:10.1093/hmg/ddr368
- Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Genetic testing for hereditary melanoma and pancreatic cancer: a longitudinal study of psychological outcome. Psychooncology. Feb 2013;22(2):276-89. doi:10.1002/pon.2080
- Lerman C, Daly M, Masny A, Balshem A. Attitudes about genetic testing for breast-ovarian cancer susceptibility. J Clin Oncol. Apr 1994;12(4):843-50. doi:10.1200/JCO.1994.12.4.843
- Sundin EC, Horowitz MJ. Impact of Event Scale: psychometric properties. Br J Psychiatry. Mar 2002;180:205-9. doi:10.1192/bjp.180.3.205
- Glanz K, Yaroch AL, Dancel M, et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol. Feb 2008;144(2):217-22. doi:10.1001/archdermatol.2007.46
- Zhang Z. Variable selection with stepwise and best subset approaches. Ann Transl Med. Apr 2016;4(7):136. doi:10.21037/atm.2016.03.35
