
Recent FDA data on enrollment to cancer therapeutic trials revealed lower trial participation rates among Black and Hispanic cancer patients relative to the cancer burden among these groups. These findings suggest the need to develop novel strategies to increase participation among racial and ethnic minority populations. This project aims to develop and implement a multi-level intervention to increase referral and recruitment of Black and Hispanic patients to NCI-sponsored therapeutic clinical trials (CTs). The ACT WONDER2S intervention will be delivered through community health educator (CHE) led outreach and navigation coupled with digital interventions for the community patient, community physician, and cancer center physician.
Links to CAB Newsletter:

Clinical research through therapeutic clinical trials is an essential approach to advancing treatments to improve patient outcomes. Yet, trial participation rates for racial and ethnic minority cancer patients is particularly low. The underrepresentation of minorities in clinical trials undermines the generalizability of clinical trial data, particularly those leading to the approval of novel cancer therapeutic agents. In this cross-sectional analysis we examined factors that may contribute to clinical trial ineligibility among Moffitt patients diagnosed with breast, lung and multiple myeloma by race/ethnicity. Our preliminary findings suggest that certain comorbidities are more prevalent in racial/ethnic minorities and could potentially impact their eligibility for trials.
It is difficult to accurately estimate KC incidence trends at the population level since US Cancer Registries are not required to report these cancers. Thus, novel methods are needed to establish population level KC registries. The goal of this project is to identify cutaneous squamous cell carcinoma (cuSCC) and basal cell carcinoma (BCC) diagnoses from electronic pathology reports at Moffitt Cancer Center. If successful, these methods could be used to inform the development of a population-based KC registries which could be used to advance KC surveillance and research.

A history of keratinocyte carcinoma (KC) has been associated with survival following the diagnosis of a subsequent primary cancer, with the direction of the association varying by subsequent cancer type. Gaining a better understanding of these patterns in KC history associated with survival with subsequent cancers can lead to identification of shared etiologic pathways that may be targeted with novel prevention and/or treatment strategies for the subsequent cancers. We sought to evaluate this association among Moffitt Cancer Center patients diagnosed and/or treated with one of six cancer types: non-Hodgkin lymphoma (NHL), breast, colon, prostate, melanoma and lung cancer.
Links to publications:

Electronic medical records (EMR) are widely used for precision medicine, observational research, and population health management. However, a large percent of EMR data can be stored in scanned documents external to the main treatment facility, posing an informatics challenge. Studies are needed to characterize the clinical information uniquely contained in scanned outside documents (SOD) to understand how access to data in SOD (or lack thereof) may affect quality reporting and the use of these “real-world data” for cancer research. The purpose of this study was to compare the completeness and concordance of two data sets abstracted with and without the use of SOD.
Links to publications:

Patients seek care at Moffitt for a variety of reasons including cancer diagnosis, cancer treatment, second opinions and receive services for non-cancerous conditions such as cancer screening and prevention. In this study, a cross-sectional analysis of more than 180,000 patients was conducted to investigate demographic and clinical factors associated with the completion of the electronic new patient clinical intake form to assess the generalizability of these data for research purposes. Additionally, in a sperate analysis using EPQ data, we examined the association between demographic characteristics and self-reported COVID-19 infection.
Links to publications:
Randomized clinical trials (RCTs) of novel treatments for solid malignancies typically measure disease progression using the Response Evaluation Criteria in Solid Tumors (RECIST) with precisely timed patient assessments relative to treatment administration. However, structured information on disease progression is not typically available in data routinely collected to monitor health status or healthcare delivery such as electronic health records, medical claims, disease registries and other patient-reported data (“real-world data”). Thus, limiting our ability to advance clinical effectiveness of cancer therapies outside of RCTs. The goal of this study was to report on current methodologic approaches to measure real-world (RW) disease progression using lung cancer as an illustrative example.
Links to publications:
Keratinocyte carcinomas (KC), including cutaneous squamous cell carcinoma (cuSCC) and basal cell carcinoma (BCC), comprise the most common group of cancers in the United States. Older age, sun exposure, and immune suppression are major risk factors for KC. Previous studies suggest that cutaneous viral infections, including cutaneous human papillomaviruses (cuHPV) and polyomaviruses (HPyV), may also be risk factors. The VIRUSCAN study was designed to investigate KC risk associated with cuHPV and HPyV, using a cohort design, multiple markers of past and recent cutaneous viral infections, and quantifiable measures of recent UVR exposure.
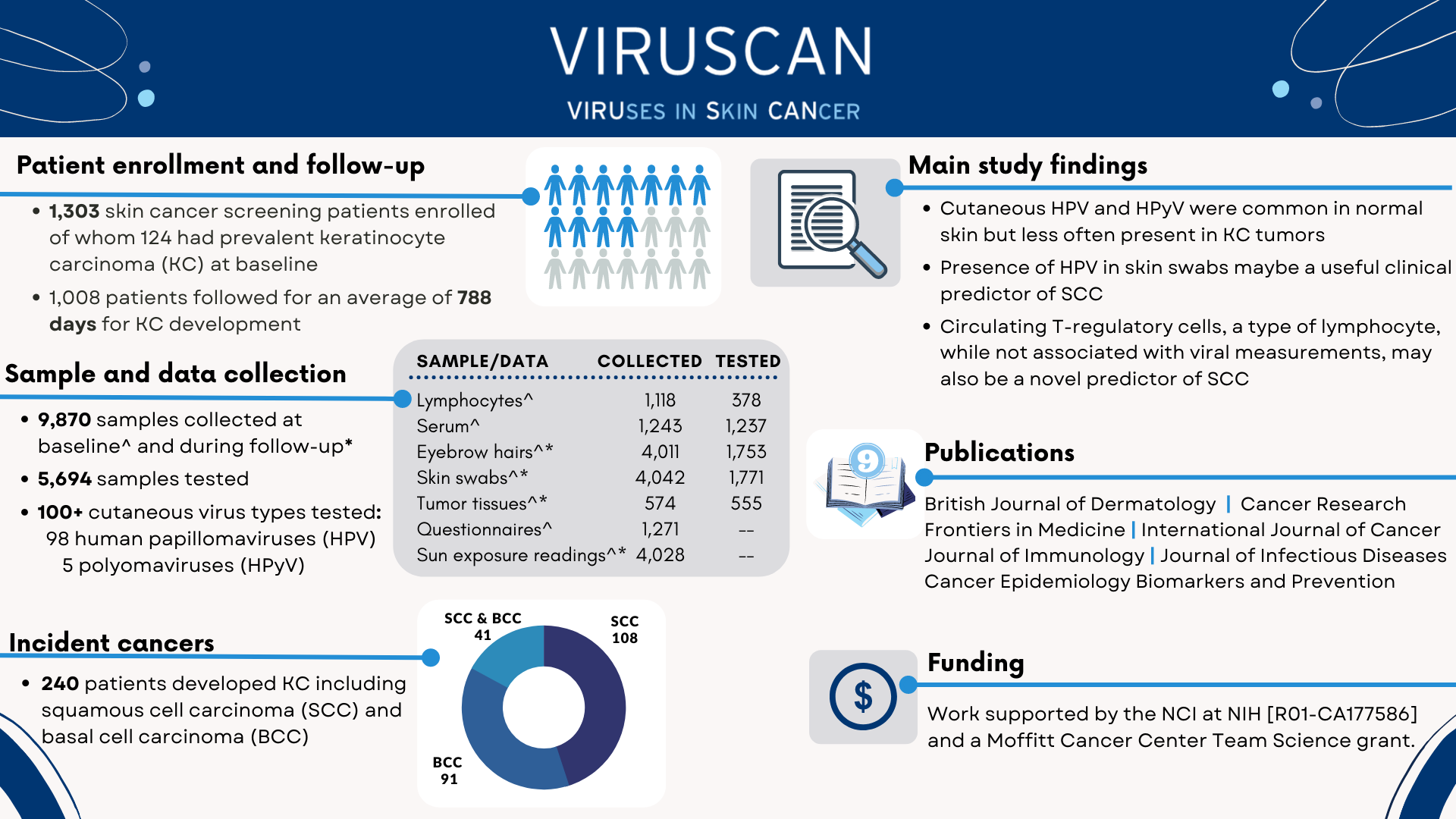
The infographic below depicts the VIRUSCAN Study high-level accomplishments.
Links to publications:
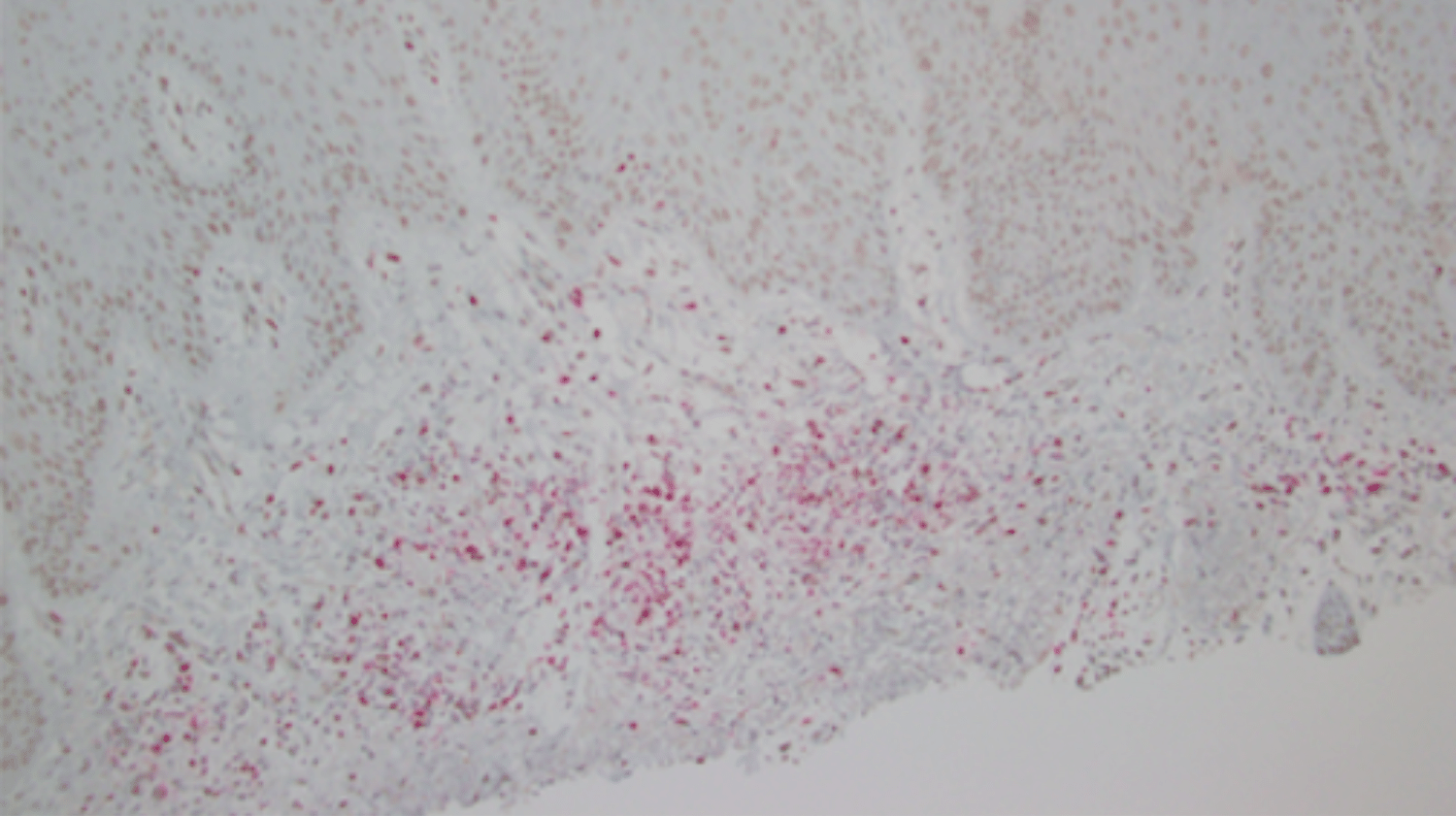
Ultraviolet radiation exposure (UVR) and immunosuppression are established risk factors for cutaneous squamous cell carcinoma (cuSCC). However UVR-related immune responses associated with cuSCC risk are not well studied. Leveraging the established VIRUSCAN cohort study designed to investigate cutaneous viral infections in KC, this work focused on studying the interplay of the immune system with UVR exposure and other KC risk factors. The project goals were to determine whether circulating T-regulatory cells are associated with increased risk of cuSCC in the context of UVR exposure.
Links to publications:
Myelodysplastic syndromes (MDS), became a reportable malignancy to population-based US cancer registries in 2001. We conducted several studies to examine possible underreporting of MDS cases to cancer registries. This work was further expanded to include etiologic and mechanistic studies, in close collaboration with immunology colleagues at Moffitt. As a greater understanding of MDS etiology will facilitate the development of new treatments and prognostication for transformation to acute myeloid leukemia.
Links to publications:
The literature pertaining to the role of human polyomavirus infections in human cancer, especially SV40, is quite controversial. While these viruses induce tumors in experimentally infected animals, they are inconsistently observed in human tumor tissues. The purpose of this research was to understand the role of human polyomavirus (JC virus [JCV], BK virus [BKV]) and simian virus 40 [SV40]) in brain tumors, lymphomas, bladder and colorectal cancer. The association between MCV and KC was also assessed in the case-control setting.
Links to publications: